UKCA extension and Amending Regulation (EU) 2023/607
Date: 08 June 2023
On 27th April 2023, the MHRA officially announced acceptance of Amending Regulation (EU) 2023/607 for MDD and AIMDD certificates as valid for placing CE-marked devices on the Great Britain market as follows:
- Class III and IIb implantable non-WET devices until 31st December 2027
- Class IIb WET, Class IIa, Class Im and Is devices until 30th June 2028
Please note that these devices are only accepted if requirements set out in (EU) 2023/607 are fully met. To benefit from these extended timelines, manufacturers must also register with the MHRA.
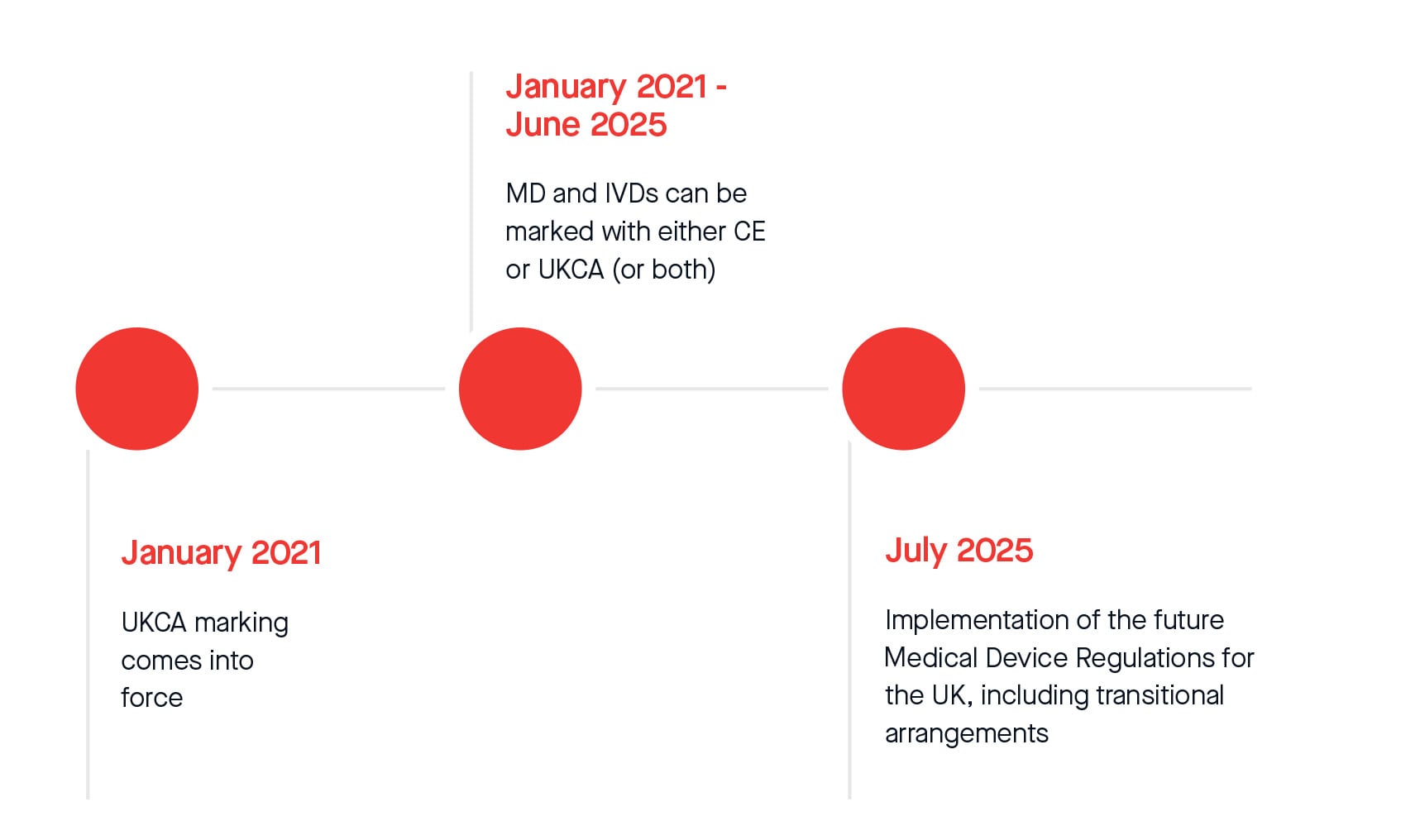
In addition, the MHRA officially announced an additional twelve-month extension to the current standstill period for compliance with UKCA marking regulations. From 1st July 2025, legislative transitional arrangements will apply for placing all Medical Devices and In-Vitro Diagnostic Medical Devices on the Great Britain market. The following represents current timelines that might be subject to change after the Statutory Instrument (S.I.) on transitional arrangements gains parliamentary approval and enters into force. The same applies to extended timelines according to Amending Regulation (EU) 2023/607.
Please see the revised transitional timeline below:
Whilst the timeline extensions bring some reassurance, we strongly urge you to submit your signed proposal and fully compliant technical documentation as soon as possible, to minimise the risk of not completing the conformity assessment in a timely manner. BSI will schedule and conduct reviews at the earliest opportunity based on the availability of reviewers.
BSI will continue to accept UKCA applications under the current legislations on a case-by-case basis, please contact your Scheme Manager for more details.
Our priority remains to maintain patient safety and ensure compliant reviews for all products within the current and future regulatory frameworks.
Further guidance can be found on GOV.UK website and our dedicated UKCA web page.
Yours sincerely,
Vishal Thakker
Head of UK Approved Body
Regulatory Services (Medical Devices), BSI


